
Definition and classification of Manure and Fertilizer Part - 2
Raghvendra Kumar Kushwaha –( Research Scholar PG)
Guru Dayal - (Field Coordinator)*
M.Sc. (Agri.) Soil Science,
Sam Higginbottom University of Agriculture,
Technology and SciencesAllahabad-211007,U.P
Corresponding author Gmail- rkk18200@gmail.com
*Self-Reliant Initiatives Through Joint Action (SRIJAN),
Regional office, Mahavir Nagar,
Manikpur, Chitrakoot, U.P.
Gmail– gurudayal20195@gmail.com
Fertilizer
Fertilizer are often manmade, inorganic compounds that provide one or more vital plant nutrients in significant quantities. The word ‘fertilizer’ is quite old. In the 1960s, the green revelation first began. More focus was placed on numerous agricultural developments, such as the use of high-quality seeds, mechanization, pesticides, and fertilizer, when the green revolution arrived. In India, M.S. Swaminathan is revered as the father of the green revolution, and Norman Borlaug is revered globally. In India, the green revolution began in 1966 to 1967, with a focus mostly on manure and fertilizer. The usage of excessive fertilizers had raised question about the fertility of the soil. Numerous scientists made the discovery of various fertilizer types while keeping this in mind. Which, together with an increase in soil fertility. The yield per hectare has significantly increased.
Classification of Fertilizer- Various scientists have offered their diverse perspectives on fertilizer classification. They are divided into different categories according to their use, which leads to a quick increase in the production per hectare. Those are listed below.
1. Straight Fertilizer-Straight fertilizer there are mostly classified into the following three section.
- Nitrogen Fertilizer
- Phosphatic Fertilizer
- Potassic Fertilizer
A. Nitrogen Fertilizer- Nitrogen fertilizer is a type of fertilizer that gives plant nitrogen .Based on the amount of nitrogen in the fertilizer, they are split into the following four classes.
- Nitrate Nitrogenous fertilizer
- Ammonical Nitrogenous fertilizer
- Ammonical and Nitrate Nitrogen Fertilizer
- Amide Nitrogen Fertilizer
a) Nitrate Nitrogenous Fertilizer- Plants that use fertilizer absorb nitrogen in the form of nitrates. They can be used for top dressing. They have an alkaline nature. These are very movable in soil, and leaching and denitrification cause their loss. Example;
- Sodium nitrate (NaNoз)- Nitrogen - 16%
Sodium- 26%
- Calcium nitrate Ca(NO3)2- Nitrogen- 15.5%
Calcium- 19%
- Potassium nitrate (KNo2)- Nitrogen- 13%
Potassium- 44%
b) Ammonical Nitrogenous fertilizer-Plants ingest nitrogen in this form, which ia acidic by nature. Denitrification and volatilization are to blame for the biggest loss. It is more soluble in soil solution and less likely to leach. Example;
- Ammonium sulphate [(NH4)2 S04]- Nitrogen- 20.5%
Sulpher- 23.24%
- Ammonium chloride (NH4Cl)- Nitrogen- 25.5%
c) Ammonical and Nitrate Nitrogen Fertilizer- Nitrate and ammonia plants absorb nitrogen from this fertilizer in the form of ammonia and nitrate. Example;
- Calcium ammonium nitrate (CAN)- Nitrogen- 25-26%
Calcium- 8.1%
Magnesium- 4.5%
- Ammonium nitrate (NH4N03)- Nitrogen- 33-26%
d) Amide Nitrogen Fertilizer-Bacteria in the soil react with amide nitrogen, converting it to ammonia and nitrate. Acidic in nature is the effect of urea on the soil. Example;
- Urea (NH2CONH2)- Nitrogen- 46%
- Calcium Cyanamid (CaCN2)- Nitrogen- 21%
Manufacturing process of urea-
2 NH3+ CO2 -----------> NH4CO2NH2 -----------> NH2CONH2 + H2O
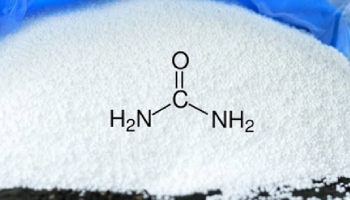
Picture 3- Structure of Urea.
Sources- Guy Sela (30 APR).
B. Phosphatic Fertilizer- P2O5 fertilizer is what plants employ as a form of Phosphatic fertilizer. Rock phosphate or phosphoric acid are the sources of Phosphatic fertilizer. The following describes how various Phosphatic fertilizers are created.
Rock Phosphate + Sulphuric acid Single Super Phosphate
Rock Phosphate + Ortho phosphoric acid Triple super phosphate
Rock Phosphate + Hydrochloric acid Di- calcium phosphate
Phosphatic fertilizer’s three key sections make up the most of it. Which are listed below;
- Water soluble phosphate fertilizer
- Citrate soluble phosphate fertilizer
- Water and Citrate soluble phosphate fertilizer
a) Water soluble phosphate fertilizer- Mono calcium phosphate and ammonium phosphate are both common types of this fertilizer. They are frequently utilised in crops with long shout periods, like as wheat, groundnut, and alkaline soil. Example;
- Single Super Phosphate- Phosphorus- 16%, Sulpher- 12%
- Double Super Phosphate- Phosphorus- 32%
- Triple Super Phosphate- Phosphorus- 46- 48%
- Di-ammonium phosphate (DAP)- Nitrogen- 18%, phosphorus- 46%.
b) Citrate soluble phosphate fertilizer- This fertilizer comes in the form of di- calcium phosphate or soluble phosphoric acid. It is utilised in long-term and acidic soil crops like sugarcane, tea, and potatoes, among other. Example;
- Basic slag- Phosphorus- 14-18%
- Di- calcium phosphate- 34-40%
c) Water and Citrate soluble phosphate fertilizer- Phosphate fertilizer that are soluble in water and citrate are only soluble in mineral acid. It is present as water and citric acid in fertilizer. They are utilised with suitable plantation crops and highly acidic soil. Example;
- Rock Phosphate- Phosphorus- 24-40%
- Row Bone meal- Nitrogen- 3-4%, phosphorus- 20-25%
- Steamed Bone meal- Phosphorus- 22-25%
C. Potassic Fertilizer- This kind of fertilizer comes in two varieties. Which are listed below.
- Potassic fertilizer with chloride
- Chloride free potassium fertilizer
a) Potassic fertilizer with chloride- A mutant form of potash should not be used to tobacco and sugarcane crops. Instead, potassium sulphate should be utilised. The least expensive potassium fertilizer is mutate od potash.
- Mutate of potash- Potassium- 58-60%
b) Chloride free potassium fertilizer- The following types of fertilizer are maintained inside it;
- Sulphate of potash- Potassium- 48-52%
- Potassium nitrate- Potassium- 44-45%
- Potassium carbonate- Potassium- 65%
|
Fertilizer Name |
Nutrient Content |
|||
|
Nitrogen % |
Phosphorus % |
Potassium % |
Other % |
|
|
Di-ammonium phosphate |
18 |
46 |
- |
- |
|
Mono ammonium phosphate |
11 |
52 |
- |
- |
|
Potassium nitrate |
13 |
- |
44 |
- |
|
Calcium nitrate |
- |
- |
- |
Ca- 19.5, Mg- 1.5 |
|
Calcium ammonium nitrate |
- |
- |
- |
Ca- 8.1, Mg- 4.5 |
|
Single super phosphate |
12 |
18.5 |
0.3 |
- |
|
Gypsum |
18.6 |
23.3 |
- |
- |
|
Sulphate of potash |
- |
- |
48-50 |
S- 17.5 |
|
Basic slag |
- |
14-18 |
- |
- |
|
Rock phosphate |
- |
24-40 |
- |
- |
Table – Different fertilizer types and the nutrients they contain.
Conversion factor
1. P = P2O5 ˟ 2.29 ----- |
| --> P
P2O5 = P ˟ 0.43 ----- |
2. K = K2O ˟ 1.20 ----- |
| --> K
K2O = K ˟ 0.83----- |
3. Ca = CaO ˟ 1.39----- |
| --> Ca
CaO = Ca ˟ 0.74----- |
4. Mn = MnO ˟ 1.29----- |
| --> Mn
MnO = Mn ˟ 0.77----- |
5. Mg = MgO ˟ 1.65----- |
| --> Mg
MgO = Mg ˟ 0.60----- |
6. Fe = FeO ˟ 1.28----- |
| --> Fe
FeO = Fe ˟ 0.77----- |
Impotent Points
- Fertility Index was given by Parker. Ramamurthy described the fertility gradient approach.
- Schoonover provided a way for the gypsum requirement.
- The best crop for green manure is thought to be Crotolaria juncea
- Green manure is produced by leguminous crops.
- Low analysis organic fertilizer is what manure is referred to as, and composting is a biological process.
- Different C:N ratio;
|
Particular |
C:N Ratio |
|
Indian soil |
8.5:1 – 12:1 |
|
Organic matter |
10 : 1 |
|
Paddy straw |
80:1 |
|
Ideal soil |
10-12:1 |
|
Farm Yard Manure |
100:1 |
|
Legumes |
10-30:1 |
|
Cultivated soil |
8:1- 15:1 |
|
Humus |
10:1 |
|
Mineralization |
< 20:1 |
|
Immobilization |
>30:1 |
|
Arable land |
8:1 – 15:1 |
|
Well decomposed compost |
10:1 |
|
Saw dust |
600:1 |
|
Compost |
< 20:1 |
- In soil that is salty or acidic, Dhaincha is used as green manure. It set nitrogen 30- 40 Kg/ha.
- Non-edible oil cakes are used as crop fertilizer, whereas edible oil cakes are utilised for feeding livestock and poultry.
- Fertilizer grade- A minimum guarantee of the amount of nitrogen, phosphorus, and potash that is available to plants. For instance, 15:15:15 (N:P: K).
- Fertilizer Ratio- How much nitrogen, phosphorus, and potash are there in total.
For instance, the fertilizer proportion will be 2:1:1 if the fertilizer grade is 12-6-6.
- Anhydrous ammonium is the highest-quality liquid ammonium fertilizer, containing 32% of nitrogen.
- Sodium nitrate, commonly known as Chilean nitrate, is the most used nitrogen fertilizer.
- Urea has an acidic effect on the soil.
- Mono-calcium phosphate is a type of fertilizer that is water soluble.
- Producer gas- This substance is a blend of Co and nitrogen. Up to 20% of Co and up to 64% of nitrogen can be found in this. It is known as producer gas.
- The Biuret concentration of urea is 0.5%, when applied topically and 1.5%, when applied tropically in soil.
- Other names for calcium ammonium nitrate include ken or farmer compost.
- The mechanism by which Calcium ammonium nitrate is formed-
 Ammonium nitrate + Limestone Calcium ammonium nitrate
Ammonium nitrate + Limestone Calcium ammonium nitrate
- The production process of Ammonium sulphate -
 Ammonium + Sulphuric acid Ammonium sulphate
Ammonium + Sulphuric acid Ammonium sulphate
- The most used phosphate fertilizer is DAP, which has an acidic character.
- The chemical name for muriate of potash is KCL, which is a member of the solanecy family.
- Sulphate of potash is used to the crops of tobacco, potatoes, coffee, tea, and onions.
- Total water soluble phosphorus in DAP is 41.6%.
- In the soil nitrogen, phosphorus, and potash should be distributed in the ratios of 4:2:1, nevertheless, in India, it is distributed in the ratio of 6.5:2.5:1.
References-
- Guy Sela (30 APR). What are urea fertilizer, urea fertilizer and how to used them without risking your crop. Agriculture, All articles, plant nutrition, soil.
- Niall Claffey (2018). What is the value of farmyard manure. Agrilland farming.








